
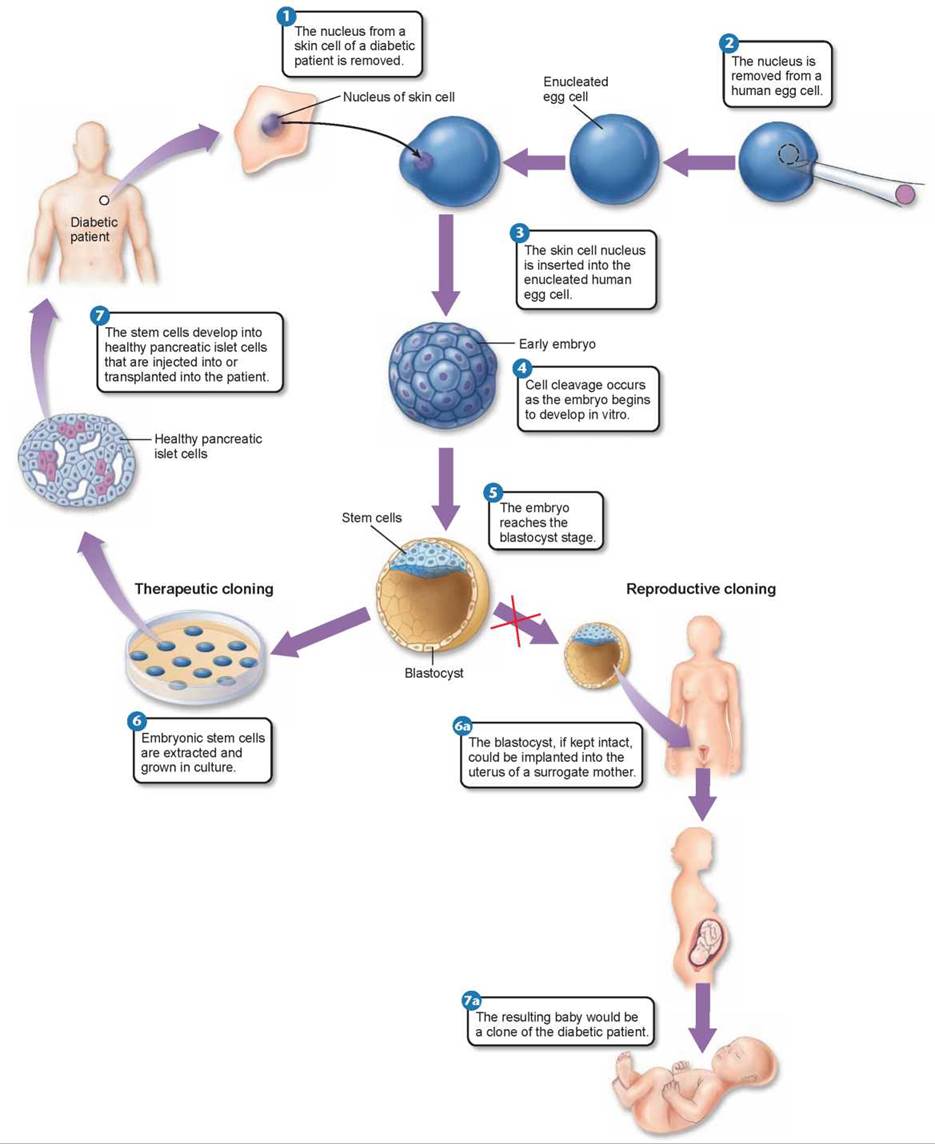
The experiments showed the capability of ESCs to produce a variety of tissues, but the results also highlight the complexity of the biological “program” of tissue development that can unfold in different biological environments.
The tissues that arise in the tumor are often advanced, organized, and complex, and include teeth, gut, hair follicles, skin, epithelium, muscle, bone, cartilage, lung tissue, and neural cells (Thompson et al., 1998). Human ESCs injected into mice form a type of benign tumor called a teratoma that is made up of tissues from all three embryonic layers. Human ESCs grown in coculture with mouse bone marrow stromal cells have been reported to produce colonies of human hematopoietic precursors and ultimately cells from the blood (Kaufman et al., 1999).įurther evidence of the multipotent capability of human ESCs is based on studies in an in vivo setting. In other experiments, cells arising from human ESCs have been reported to express genes associated with liver and pancreas function (Schuldiner et al., 2000).
Cells derived from human embryonic bodies include “rhythmically contracting cardiomyocytes, pigmented and nonpigmented epithelial cells, and neural cells displaying an exuberant outgrowth of axons and dendrites” (Odorico et al., 2001). Evidence of the differentiation in culture includes detection of the products of genes associated with different cell types and in some cases by the characteristic shapes that are peculiar to different cell types. When removed from feeder cells and grown in suspension (in liquid), human ESCs form aggregated balls of cells called “embryonic bodies,” which have been reported to give rise to a multiplicity of cell types representing all three layers of embryonic tissue development (Itskovitz-Eldor et al., 2000 Reubinoff et al., 2000 Schuldiner et al., 2000). Such in vitro culturing presents certain theoretical hazards to the use of stem cells for regenerative medicine, such as the spread of viruses and other infectious agents not normally found in humans. The mechanism of how feeder cells maintain the proliferation of undifferentiated ESCs is unknown. The feeder cells are inactivated, so they are not dividing and expanding, but they produce growth factors that sustain the ESCs. Mouse ESCs will proliferate in an undifferentiated state in the presence of a biochemical called leukemia inhibitory factor (LIF), but the culture conditions required to keep human ESCs from differentiating include growing them in petri dishes on a layer of mouse embryonic fibroblasts (referred to as “feeder cells”) in a medium containing serum from cows. Among the types of cells derived from cultured mouse ESCs are fat cells, various brain and nervous system cells, insulin-producing cells of the pancreas, bone cells, hematopoietic cells, yolk sac, endothelial cells, primitive endodermal cells, and smooth and striated muscle cells, including cardiomyocytes-heart muscle cells (Odorico et al., 2001).Įxperience with mouse ESCs has provided clues to methods for culturing human ESCs and leading them to differentiate. The factors that permit the mouse ESC to continue replicating in the laboratory without differentiation and methods to trigger differentiation into different cell types that exhibit normal function have been actively explored. Nevertheless, ESCs derived from mouse blastocysts have been studied for 2 decades and provide a critical baseline of knowledge about the biology and cultivation of these cells (Torres, 1998 Wobus and Boheler, 1999). Careful monitoring of the aging ESC lines will be needed to evaluate the significance of genetic changes that are expected to occur over time.īecause human ESCs have only recently become available for research, most of what is known about ESCs comes from studies in the mouse, which, as noted in Chapter 2, cannot be presumed to provide definitive evidence of the capabilities of human cells. Human ESCs that have been propagated for more than 2 years also demonstrate a stable and normal complement of chromosomes, in contrast to the unstable and abnormal complement of embryonic cancer cell lines used in the past to study early stages of embryonic development. As indicated at the workshop by Thomas Okarma and Ron McKay, ESC lines established from single cells have been demonstrated to proliferate through 300-400 population-doubling cycles. Under appropriate culture conditions, ESCs have demonstrated a remarkable ability to self-renew continuously, that is, to produce more cells like themselves that are multipotent. Human ESCs were successfully grown in the laboratory for the first time in 1998 (Thompson et al., 1998). PROPERTIES OF ESCs IMPORTANT FOR REGENERATIVE MEDICINE


 0 kommentar(er)
0 kommentar(er)
